Regulatory vs. Voluntary Industry Approaches in ‘Conflicts of Interest Disclosure’ Systems across Borders
By Geislar, S.; Mintzes, B.; Holman, B.; Karanges, E.; Dai-Keller, Z.; Chiu, K.; Sung, R., Flood, L.
Abstract
The Affordable Care Act Section 6002, also known as the Sunshine Act, requires pharmaceutical companies to disclose payments to physicians which are then publicly available on the Open Payments (OP) system website. The OP is meant to provide transparency on financial ties with pharmaceutical companies which previous research has shown can affect the design, conduct, and reporting of clinical trials of drug treatments [1]. Since 2015, a similar system has been in place under the auspices of Medicines Australia (MA), Australia’s research-based pharmaceutical industry association. MA requires member countries to report payments provided to individual clinicians.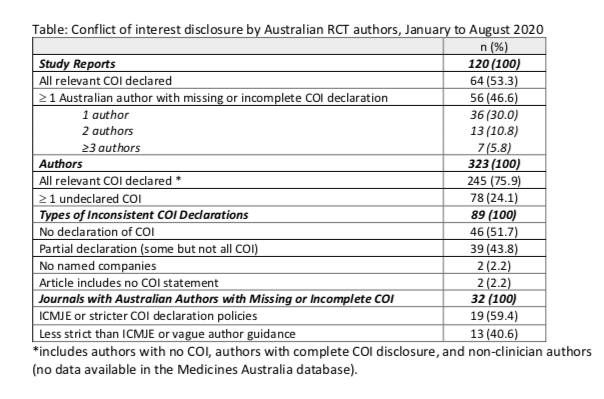
Although disclosure does not eliminate conflicts of interest (COI), it allows readers and reviewers to consider potential impacts. Unfortunately, the research journals where the studies are published rely on self-reported disclosures by authors, rather than information in the databases.
The current study examines how information about industry financial ties is managed across national borders and with different approaches (i.e., regulatory vs. voluntary industry action). We first compare the accuracy and completeness of US and Australian authors’ self-reported COI against the relevant payments database. We then conduct two comparative analyses examining: 1) consistency of journals’ author instructions with ICMJE standards; and 2) whether differences in the structure and criteria of the two transparency systems (e.g., regulatory vs. voluntary industry action, types of payments disclosed) shape apparent failure and success.
The sample of articles was selected from Medline. Search criteria included publication data between Jan - Aug 2020, RCT study design, at least one ‘Australia’ author affiliation, and at least one US-affiliated author. RCTs testing prescription-only medicines and vaccines in clinical populations were included. Of the 583 unique articles that met the RCT and publication date inclusion criteria, 120 had at least one Australian author (author n = 323), and 67 of those articles contained at least one US author (author n = 465).
To assess whether the apparent success or failure of the transparency tools is shaped by the features of the databases themselves, we compare the default payment classes listed by the OP and the MA. For instance, whereas MA does not include “food and beverage” payments, these payment types constitute one of the most frequent payments to physicians in the OP database. In this case, by controlling for payment type, we can assess whether and to what extent the apparent success or failure of one transparency system is explained by discrepancies in the structure of the system itself (i.e., inadequacies of the payment measures).
Preliminary results point to the inadequacy of author self-report in the context of industry financial ties, the importance of content validity in monitoring payment types, as well as the inadequacy of coverage in voluntary industry action approaches.
These shortcomings highlight the need for more transparent and comprehensive COI reporting in venues where research findings are actually encountered (e.g., research publications). Overall, this study aims to highlight the strengths and weaknesses of current transparency tools in COI reporting and knowledge management systems more broadly.
References
1. Fabbri A, Lai A, Grundy Q, Bero LA. The influence of industry sponsorship on the research agenda: a scoping review. Am J Public Health2018;108:e9-16. doi:10.2105/AJPH.2018.304677 pmid:30252531
2. Taheri C, Kirubarajan A, Li X, et al. Discrepancies in self-reported financial conflicts of interest disclosures by physicians: a systematic review. BMJ Open 2021;11:e045306. doi:10.1136/ bmjopen- 2020-045306
3. Rasmussen K, Schroll J, Gøtzsche PC, Lundh A. Under-reporting of conflicts of interest among trialists: a cross-sectional study. J R Soc Med. 2015 Mar;108(3):101-7. doi: 10.1177/0141076814557878.
Contributors:Geislar, S.; Mintzes, B.; Holman, B.; Karanges, E.; Dai-Keller, Z.; Chiu, K.; Sung, R., Flood, L.